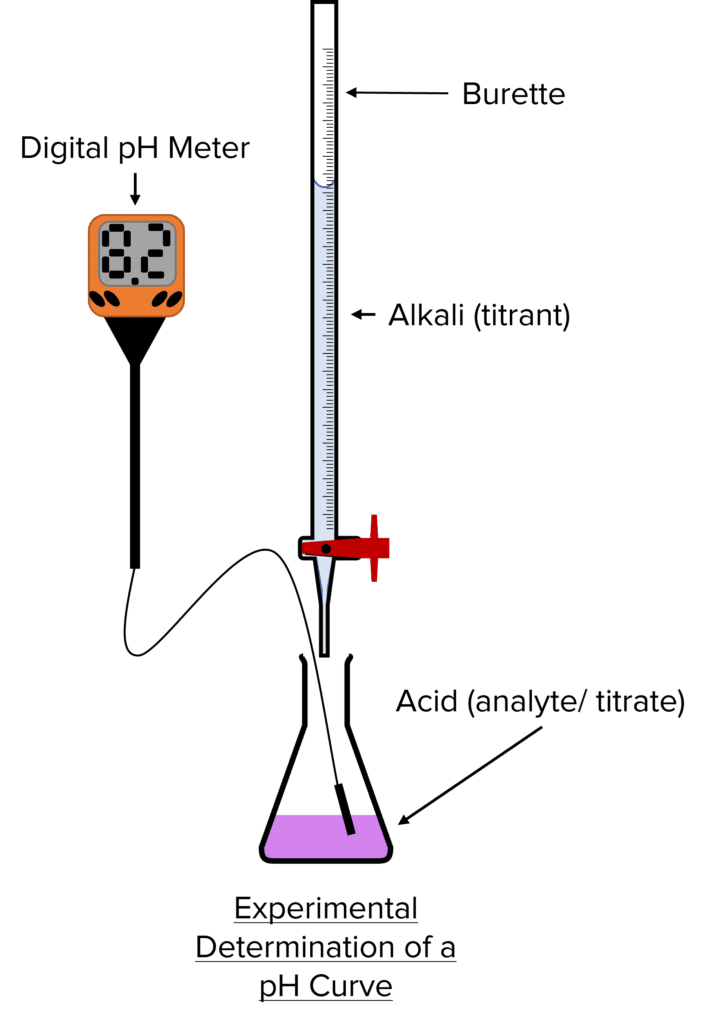
Define Indicator Used In Titration . Indicators are substances whose solutions change color due to changes in ph. The indicator used is dichlorofluorescein which acts as an anion in solution. In chemistry, an indicator is defined as a substance that undergoes a distinct observable change when the conditions of its solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Indicators are substances that change colour when they are added to acidic or alkaline solutions; As you begin to titrate a chemical solution,. They are usually weak acids or bases,. In chloride solution, due to excess.
from mmerevise.co.uk
In chemistry, an indicator is defined as a substance that undergoes a distinct observable change when the conditions of its solution. The indicator used is dichlorofluorescein which acts as an anion in solution. Indicators are substances that change colour when they are added to acidic or alkaline solutions; As you begin to titrate a chemical solution,. They are usually weak acids or bases,. In chloride solution, due to excess. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Indicators are substances whose solutions change color due to changes in ph. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes.
Titrations and Uncertainties MME
Define Indicator Used In Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. In chloride solution, due to excess. Indicators are substances that change colour when they are added to acidic or alkaline solutions; They are usually weak acids or bases,. The indicator used is dichlorofluorescein which acts as an anion in solution. As you begin to titrate a chemical solution,. In chemistry, an indicator is defined as a substance that undergoes a distinct observable change when the conditions of its solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Indicators are substances whose solutions change color due to changes in ph.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Define Indicator Used In Titration Indicators are substances whose solutions change color due to changes in ph. As you begin to titrate a chemical solution,. Indicators are substances that change colour when they are added to acidic or alkaline solutions; In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Titration is the slow addition of one. Define Indicator Used In Titration.
From www.savemyexams.co.uk
Titrations (1.2.9) DP IB Chemistry SL Revision Notes 2016 Save My Define Indicator Used In Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions; They are usually weak acids or bases,. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Indicators are substances whose solutions change color due to changes in ph. In chloride solution, due to excess. Titration is. Define Indicator Used In Titration.
From solutionpharmacy.in
Complexometric Titration Solution Parmacy Define Indicator Used In Titration In chloride solution, due to excess. As you begin to titrate a chemical solution,. They are usually weak acids or bases,. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In chemistry, an indicator is defined as a substance that undergoes a distinct observable change. Define Indicator Used In Titration.
From www.youtube.com
Question 17. Define EDTA titration and types of complexometric Define Indicator Used In Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. As you begin to titrate a chemical solution,. In chemistry, an indicator is defined as a substance that undergoes a distinct observable change when the conditions of its solution. In chloride solution, due to excess. In. Define Indicator Used In Titration.
From www.numerade.com
SOLVEDUse Figure 19.6 to find an indicator for these titrations (a) 0 Define Indicator Used In Titration In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Indicators are substances that change colour when they are added to acidic or alkaline solutions; In chloride solution, due to excess. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases,. In chemistry,. Define Indicator Used In Titration.
From slideplayer.com
Indicators. ppt download Define Indicator Used In Titration Indicators are substances whose solutions change color due to changes in ph. In chemistry, an indicator is defined as a substance that undergoes a distinct observable change when the conditions of its solution. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Indicators are substances that change colour when they are. Define Indicator Used In Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Define Indicator Used In Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions; The indicator used is dichlorofluorescein which acts as an anion in solution. In chemistry, an indicator is defined as a substance that undergoes a distinct observable change when the conditions of its solution. Indicators are substances whose solutions change color due to changes in ph.. Define Indicator Used In Titration.
From www.vrogue.co
Acid Base Titration With Phenolphthalein Indicator Wo vrogue.co Define Indicator Used In Titration The indicator used is dichlorofluorescein which acts as an anion in solution. Indicators are substances whose solutions change color due to changes in ph. In chloride solution, due to excess. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In chemistry, an indicator is defined. Define Indicator Used In Titration.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Define Indicator Used In Titration The indicator used is dichlorofluorescein which acts as an anion in solution. They are usually weak acids or bases,. In chloride solution, due to excess. In chemistry, an indicator is defined as a substance that undergoes a distinct observable change when the conditions of its solution. Indicators are substances whose solutions change color due to changes in ph. Titration is. Define Indicator Used In Titration.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Define Indicator Used In Titration As you begin to titrate a chemical solution,. They are usually weak acids or bases,. The indicator used is dichlorofluorescein which acts as an anion in solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Indicators are substances whose solutions change color due to. Define Indicator Used In Titration.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Define Indicator Used In Titration Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Indicators are substances that change colour when they are added to acidic or alkaline solutions; As you begin to titrate a chemical solution,. They are usually weak acids or bases,. In chloride solution, due to excess.. Define Indicator Used In Titration.
From www.chegg.com
Solved Define the following terms titration indicator Define Indicator Used In Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions; In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. The indicator used is dichlorofluorescein which acts as an anion in solution. In chemistry, an indicator is defined as a substance that undergoes a distinct observable change. Define Indicator Used In Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Define Indicator Used In Titration Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases,. Indicators are substances that change colour when they are added to acidic or alkaline solutions; In chemistry, an indicator is defined as a substance that undergoes a distinct observable change when the conditions of its solution. In chloride solution, due to. Define Indicator Used In Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Define Indicator Used In Titration Indicators are substances whose solutions change color due to changes in ph. As you begin to titrate a chemical solution,. In chloride solution, due to excess. They are usually weak acids or bases,. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. In chemistry, an indicator is defined as a substance. Define Indicator Used In Titration.
From mungfali.com
Acid Base Titration Indicator Define Indicator Used In Titration In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. In chloride solution, due to excess. They are usually weak acids or bases,. The indicator used is dichlorofluorescein which acts as an anion in solution. As you begin to titrate a chemical solution,. In chemistry, an indicator is defined as a substance. Define Indicator Used In Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Define Indicator Used In Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions; They are usually weak acids or bases,. In chemistry, an indicator is defined as a substance that undergoes a distinct observable change when the conditions of its solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a. Define Indicator Used In Titration.
From www.studyxapp.com
7 a mohr titration is a precipitation titration used to analyze the Define Indicator Used In Titration They are usually weak acids or bases,. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Indicators are substances whose solutions change color due to changes in ph. The indicator used is dichlorofluorescein which acts as an anion in solution. As you begin to titrate a chemical solution,. In chloride solution,. Define Indicator Used In Titration.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Define Indicator Used In Titration Indicators are substances that change colour when they are added to acidic or alkaline solutions; As you begin to titrate a chemical solution,. In many titrations, you use a chemical called an indicator, which lets you know when the titration finishes. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Define Indicator Used In Titration.